Laboratories at SFSU generate both non-infectious (Risk Group-1) and some infectious or potentially infectious (Risk Group-2) waste as part of their research and teaching operations.
Biowaste Storage and Disposal Protocols
The protocols for handling, storing, and disposing of biological waste are available in the drop down menu below. To assist lab personnel with the variety of biological waste generated, a downloadable flow chart is available.
Quick Links
Most of the biological waste generated by SFSU laboratories are at Risk Level 1 (RG-1), requiring standard microbiological practices at Biosafety Level 1 (BSL-1). These materials are pose a zero to low risk of infection to lab personnel, the community or the environment. Waste generated is NOT subject to the California Medical Waste Management Act (MWMA). Practices have been established at SFSU to differentiate BSL-1 waste storage (non-biohazardous) and biohazardous (BSL-2) storage that IS subject to the MWMA.
At SFSU, storage time is limited to 30 days for BSL-1 waste, unless it is frozen.
SFSU laboratories do generate some biohazardous or "medical" waste (RG-2) that is subject to the MWMA. RG-2 agents and materials usually required Biosafety Level 2 containment practices.These must be stored in red biohazard bags inside hard-sided containers that meet MWMA requirements.
The SFSU Biohazard Safety Program describes the storage, handling, treatment and disposal procedures. These procedures are detailed and meet the requirements listed in the SFSU Medical Waste Management Plan.
The Biology Department is approved to treat biohazardous/medical waste and the autoclave locations are listed in the SFSU Medical Waste Management Plan.
Storage Time Limits: The MWMA specifies that BSL-2 waste may be stored up to 7 days in the lab. If kept frozen, the waste may be stored up to 90 days.
EH&S has established a Medical Waste Management Plan as required by the CA MWMA and the City/County of San Francisco. This plan is periodically reviewed during scheduled inspections.
Practices for the collection, treatment and disposal of laboratory biohazardous and pathology waste must meet the requirements described in the SFSU Medical Waste Management Plan.
Contact: Juliana Cayetano, EH&S
EH&S has established a written biosafety program as required by National Institutes of Health (NIH) and Centers for Disease Control (CDC) guidelines. It describes the biosafety program, required safe work practices, and labeling, storage, treatment, and disposal of biological materials in laboratories.
Contact: Linda Vadura, EH&S
Sharps waste includes needles, syringes, hard pipettes, razor blades, cutting tools and other items that can cut a user or puncture a bag. Such items, if they are or appear to be laboratory tools, should be collected in a Sharps container. This includes both BSL-1 and BSL-2 contaminated items and un-contaminated laboratory tools like this.
Sharps from biological processes may continue to accumulate until the container is full or no longer needed. There is no maximum accumulation time.
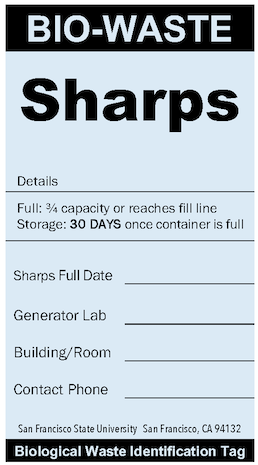
Once full, sharps containers must be transferred to the "biohazardous waste collection room" for your department within 30 days.
Chemical-Only Applications
Note that used laboratory sharps used to inject chemicals into analytical equipment are actually chemical hazardous waste and must be labeled and handled accordingly.
Biological Waste Identification Tags
Bio-Waste ID Tag
This is a double-sided tag. Fill out the correct side for the waste classification.

Sharps Container ID Tag
Sharps waste from laboratory biological operations. Both BSL-1 and BSL-2 sharps waste containers should use this tag. SFSU and the waste contractor do not differentiate sharps waste for disposal.